
IQIRVO ELATIVE trial
Safety data
IQIRVO safety data: a 2L treatment for PBC with a similar
Any AE attributed to the ELATIVE trial regimen that emerged during treatment period*1–3
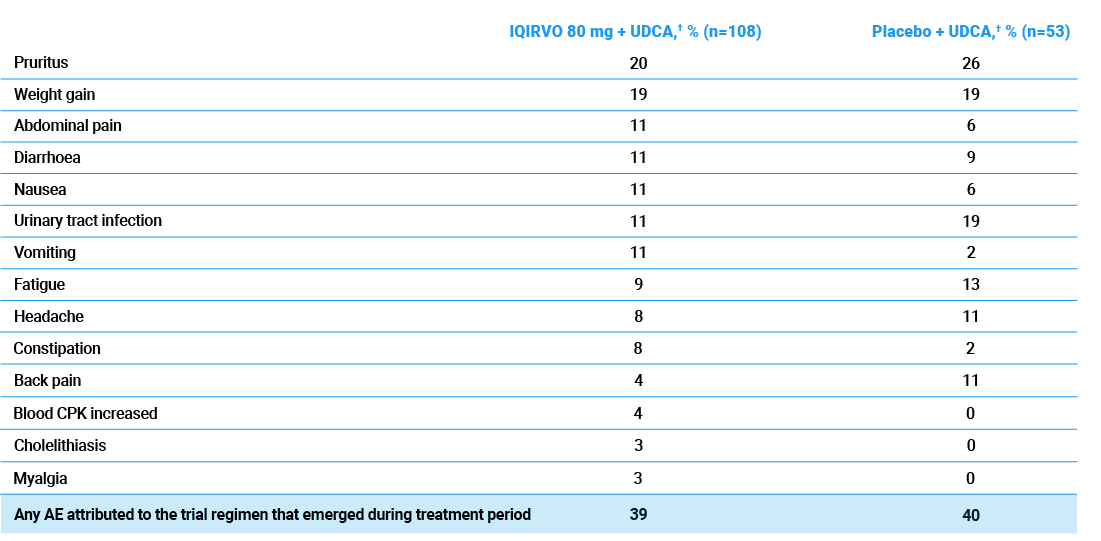
- Most AEs were mild to moderate1,2
- Serious TEAEs were similar to placebo + UDCA (10% vs 13%, respectively)1
- Discontinuation rate due to AEs was similar to placebo + UDCA (10% vs 9%, respectively)1
- The most common AE leading to discontinuation was increased blood CPK (3.7%)2
Full safety information can be found in the SmPC.
Footnotes
*Included are any AEs occurring in >10% of patients in either treatment group in the ELATIVE trial, as well as those listed as common (≥1/100 >1/10) in the SMPC for completeness.1,2
†Patients either received IQIRVO on a background of UDCA (102/108, 94%) or received UDCA plus a placebo (51/53, 96%).1
Abbreviations
AE, adverse event; CPK, creatine phosphokinase; TEAE, treatment-emergent adverse event; UDCA, ursodeoxycholic acid.
References
- Kowdley KV et al. N Engl J Med. 2024;390(9):795–805.
- IQIRVO® (elafibranor) Summary of product characteristics (SmPC). 2024.
- Kowdley KV et al. Supplement to: N Engl J Med. 2024;390(9):795–805.
Resources
- PBC
- IQIRVO MoA
- The ELATIVE trial: Study design & baseline characteristics
- The ELATIVE trial: Efficacy
- The ELATIVE trial: Safety data
- IQIRVO Safety Data
- Dosing
- Resources
