Abbreviations
PBC, primary biliary cholangitis; PPAR α/δ, peroxisome proliferator-activated receptor alpha + delta
Reference
- IQIRVO® (elafibranor) Summary of product characteristics (SmPC). 2024.
This section of the website is for UK Healthcare Professionals only. If you are not a Healthcare Professional, please click here.
This website has been commissioned by Ipsen UK and is intended for a UK audience.

Mechanism of action
While the exact mechanism by which IQIRVO exerts its therapeutic effects in patients with PBC is not fully understood, it is thought that PPAR-α and PPAR-δ are key regulators of bile acid homeostasis, inflammation and fibrosis.

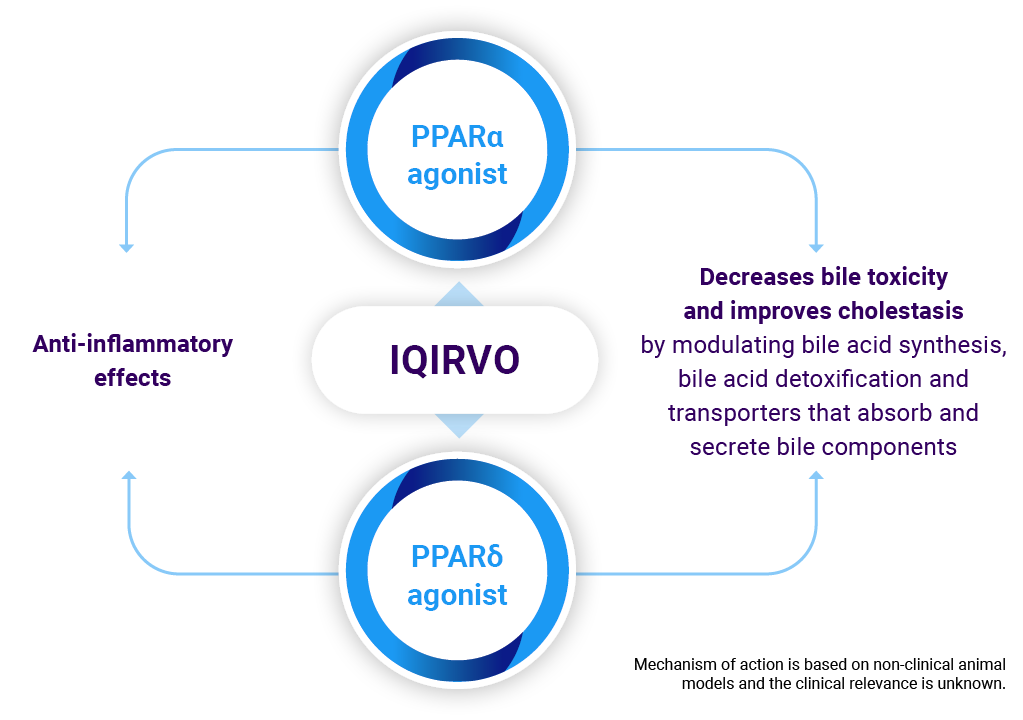
PBC, primary biliary cholangitis; PPAR α/δ, peroxisome proliferator-activated receptor alpha + delta
▼This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. Reporting of side effects. If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the Yellow Card Scheme at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in Google Play or Apple App Store. Side effects should also be reported to Ipsen via email at pharmacovigilance.uk-ie@ipsen.com or phone 01753 627777. By reporting side effects, you can help provide more information on the safety of this medicine.