
SUMMARY
Cabozantinib Ipsen, in combination with nivolumab, is indicated for the first-line treatment of advanced renal cell carcinoma in adults1.
The CheckMate 9ER trial is a phase 3, randomised, open-label trial assessing Cabozantinib + nivolumab vs. sunitinib in patients with previously untreated advanced renal cell carcinoma (aRCC).
Early tumour control with sustained long-term overall survival demonstrated by Cabozantinib + nivolumab vs sunitinib, in the 68-month CheckMate 9ER follow-up, in patients with previously untreated advanced renal cell carcinoma (aRCC).2
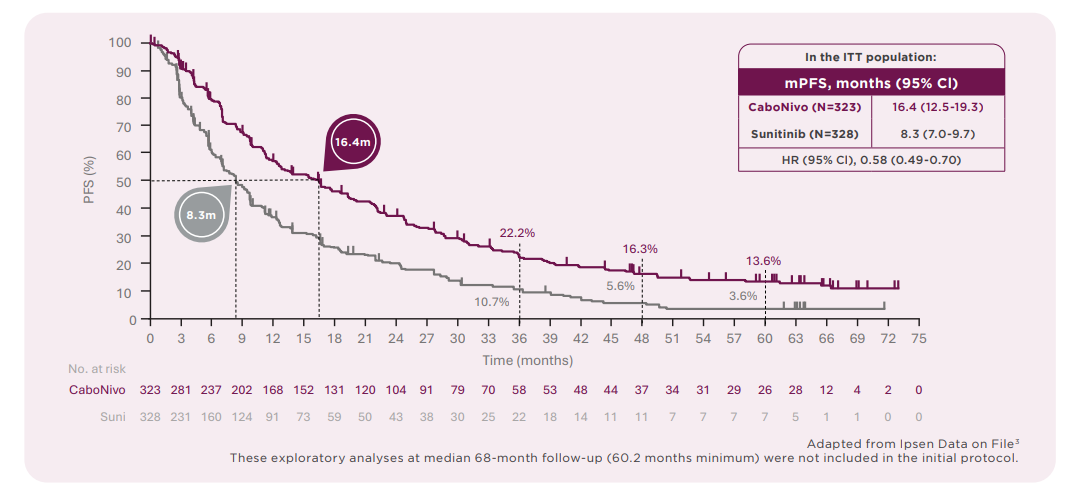
Sustained long-term overall survival at 67.6 months versus sunitinib2
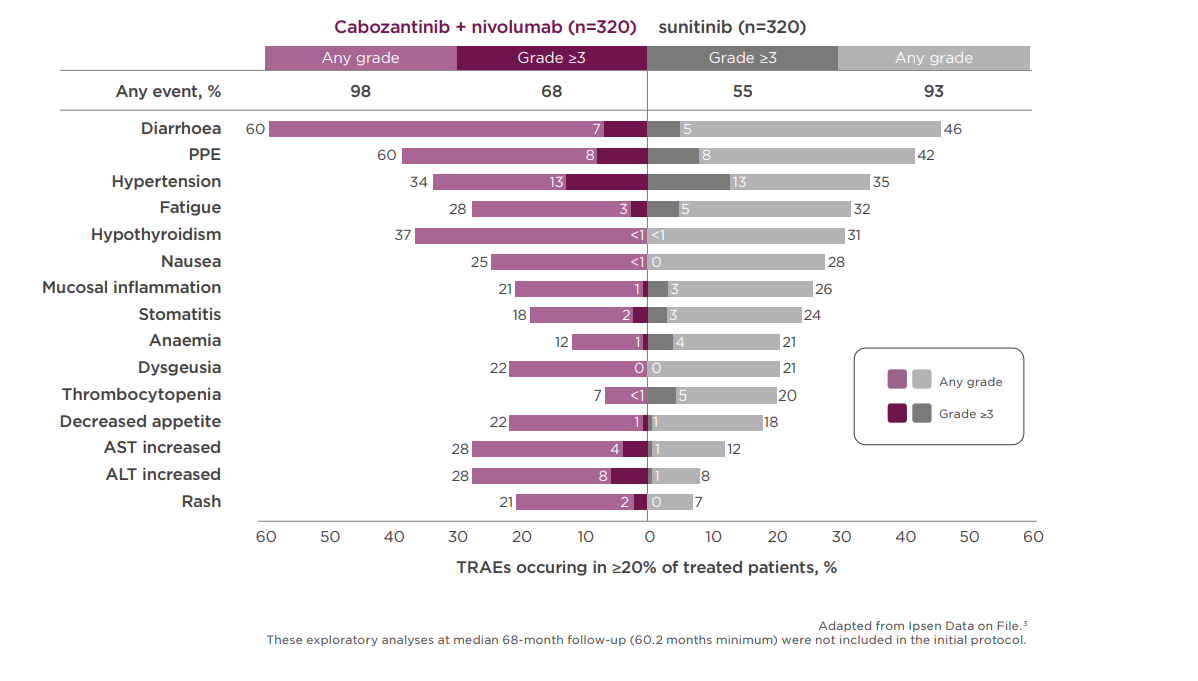
Established patient safety and tolerability profile2
An established safety profile with no new safety signals compared with the previous cut-off was seen in the 67.6-month follow-up2
Discontinuation rates due to TRAEs with Cabozantinib + nivolumab were 6% simultaneously and 1% sequentially, 11% for Cabozantinib alone, 10% for nivolumab alone and 11% with sunitinib.2
One tablet, once a day with adjustable dosing1
Cabozantinib is taken as one tablet, once daily with a recommended starting dose of 40 mg when administered in combination with nivolumab.1 Management of suspected adverse drug reactions may require temporary treatment interruption and/or dose reduction.1
Dosing can be reduced to 20 mg once daily, then 20 mg every other day to manage TRAEs, if required.1
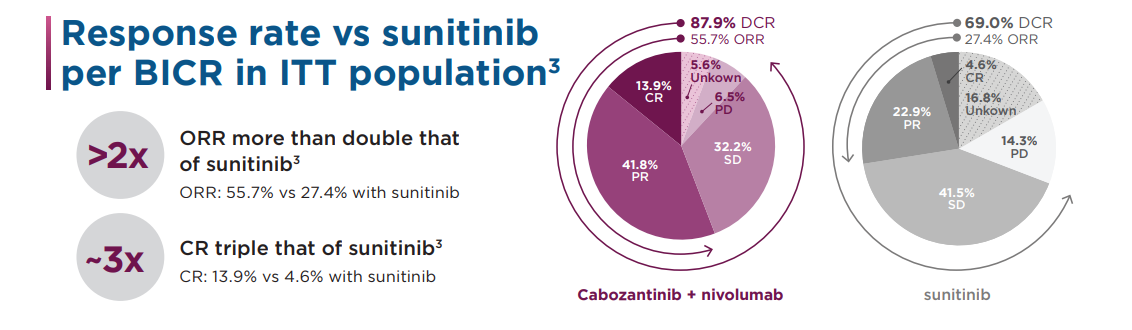
‡ Disease control calculated as patients in ITT population achieving CR + PR + SD (CR: 13.9%; PR: 41.8%; SD: 32.2%); §If required.
Abbreviations:
VEGF, vascular endothelial growth factor; IO, immunotherapy; TKI, tyrosine kinase inhibitor; aRCC,
advanced renal cell carcinoma; mPFS, median progression-free survival; HR, hazard ratio; CI,
confidence interval; ORR, objective response rate; CR, complete response; ITT, intention-to-treat;
mOS, median overall survival; TRAE, treatment-related adverse event.
References:
1. Cabozantinib Summary of Product Characteristics.
2. Motzer RJ, et Al.. Final analysis of nivolumab plus cabozantinib for advanced renal cell carcinoma from the randomized phase III CheckMate 9ER trial. Ann Oncol. 2025 Sep 23:S0923-7534(25)04714-3.
CBZ-UK-000875 | November 2025
